Appel Reaction
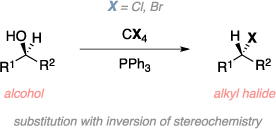
The Appel reaction is an organic reaction used to convert an alcohol to an alkyl halide using a tetrahalomethane and triphenylphosphine. The reaction begins with the halogenation of triphenyl-phosphine followed by the formation of the alkoxide from the alcohol starting material. The alkoxide subsequently attacks the phosphorous, releasing the halide leaving group. In a nucleophilic substitution reaction (SN2), the halide (nucleophile) attacks the carbon stereocenter resulting in the the final alkyl halide product with inverted stereochemistry. Triphenylphosphine oxide is a byproduct of this reaction and the formation of the strong P=O double bond is a driving force for this reaction.[1]
Mechanism

References:
| 1. |
Appel, R.
Angew. Chem. Int. Ed. Engl.
1975,
14,
801–811.
|