Kumada cross-coupling
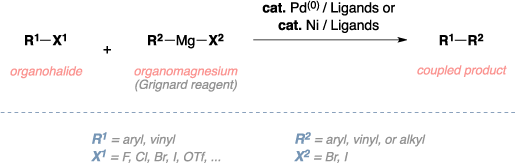
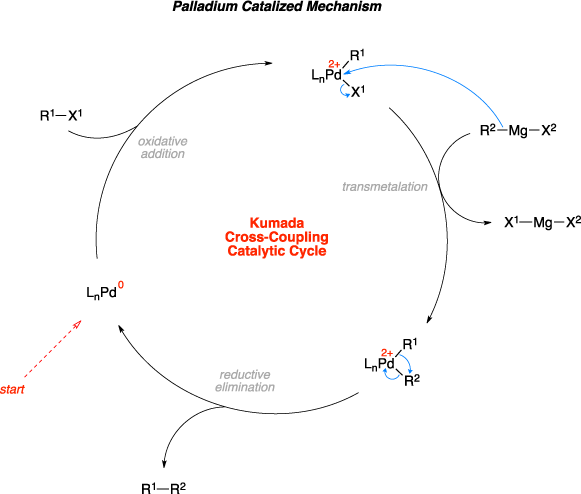
The Kumada cross-coupling reaction is the organic reaction of an organohalide with an organomagnesium compound, also known as a Grignard reagent, to give the coupled product using a palladium or nickel catalyst. The palladium catalyzed mechanism begins with oxidative addition of the organohalide to the Pd(0) to form a Pd(II) complex. Transmetalation with the Grignard reagent then follows, where the R group of the Grignard reagent replaces the halide anion on the palladium complex and makes a magnesium(II) halide salt. Reductive elimination then gives the final coupled product, regenerates the catalyst, and the catalytic cycle can begin again.[1]
Mechanism
References:
| 1. |
Tamao, K.; Sumitani, K.; Kumada, M.
J. Am. Chem. Soc.
1972,
94,
4374–4376.
|