Fischer indole synthesis
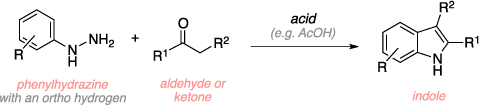
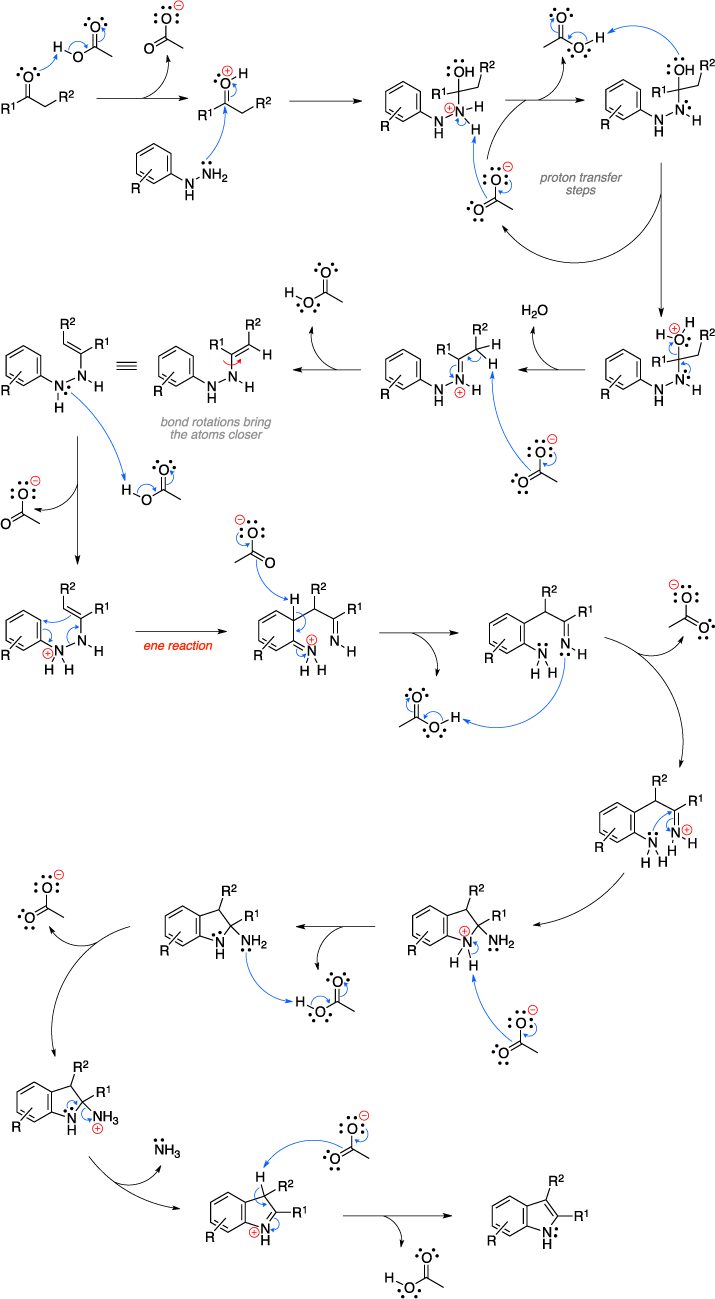
The Fischer indole synthesis is an organic reaction used to convert a phenyl hydrazine and an aldehyde or ketone to an indole using an acid catalyst. The mechanism begins with formation of a phenylhydrazone through the acid catalyzed reaction of the hydrazine with the carbonyl. The phenylhydrazone then rearranges to the enamine and gets protonated on the phenyl nitrogen. An "ene reaction" (3,3-sigmatropic rearrangement) ensues, resulting in a diimine and loss of aromaticity. Additional key steps include rearomatization, formation of a cyclic aminal, and the expulsion of ammonia to give the indole product.[1][2]
Mechanism
References:
| 1. |
Fischer, E.; Jourdan, F.
Ber. Dtsch. Chem. Ges.
1883,
16,
2241–2245.
|
| 2. |
Fischer, E.; Hess, O.
Ber. Dtsch. Chem. Ges.
1884,
17,
559–568.
|