Pictet-Spengler reaction
Also known as: Pictet-Spengler tetrahydroisoquinoline synthesis

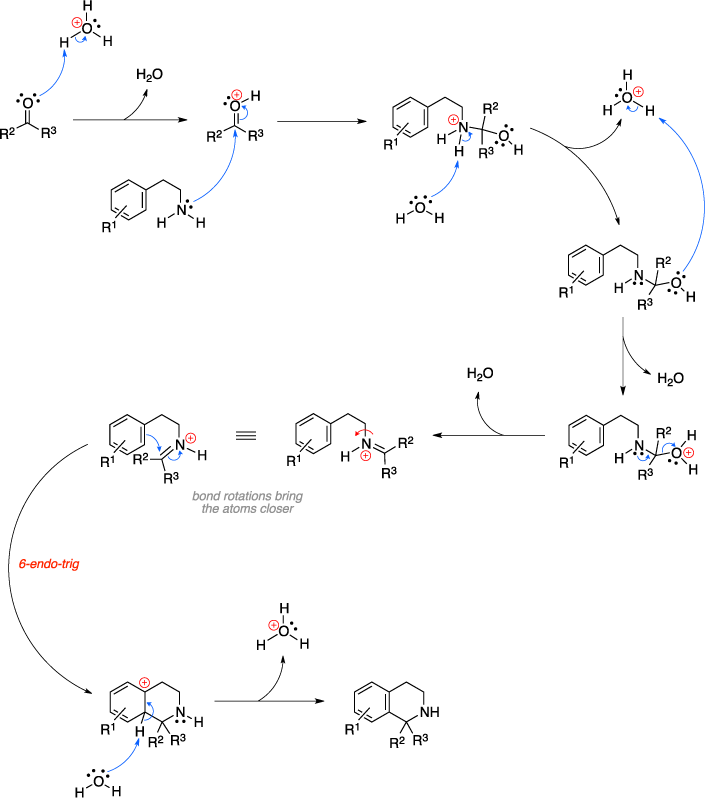
The Pictet-Spengler reaction is an organic reaction used to convert a β-arylenylamine and an aldehyde or ketone to a tetrahydroisoquinoline using an acid catalyst. The mechanism begins with protonation of the carbonyl oxygen by the acid which is subsequently attacked by the amine reagent. Proton transfer steps and the release of a water molecule results in a protonated imine intermediate, which then undergoes a 6-endo-trig cyclization reaction with loss of aromaticity of the aryl ring. A final deprotonation step restores the aromaticity and results in the tetrahydroisoquinoline product.[1]
Mechanism
References:
| 1. |
Pictet, A.; Spengler, T.
Ber. Dtsch. Chem. Ges.
1911,
44,
2030–2036.
|